Prospective Investor Message
MezLight will replace the surgeon's headlight as a superior solution for precision lighting in the operating room. This will result in less pain & injury for surgeons while decreasing the chances for surgical site infection in patients.
Who is Mezlight?
Roughly 5 years ago our neighbor, Dr Josh Mezrich, transplant surgeon from the UW, was visiting on our porch. He was complaining about his back and his wife’s back. Gretchen Schwarze is a vascular surgeon, also at the UW. He told us about his idea for a snake light to be used in the operating room (OR) that would attach to the rails; it needed to be sterile; and most importantly have the same intensity as the industry standard surgeon headlight. Josh basically desired to take the headlight off of the surgeon’s head and give the surgeon better light, better flexibility, and best of all give the surgeon relief of head/neck pain directly caused by the headlight. That became the concept for MezLight. Gretchen, Josh, Craig and I self-funded the initial market analysis and the first rounds of design and prototype build.
Several very important things came to light, pun intended, while we went through the beginning of design. On July 11, 2017 the FDA announced a list of class II devices that the agency determined to no longer require pre-market notification to provide reasonable assurance of safety and effectiveness. These exemptions decreased regulatory burdens on the medical device industry by eliminating private costs and expenditures required to comply with Federal regulation. Specifically, for these exempted class II devices, regulated industry no longer has to invest time and resources in pre-market notifications, including preparation of documents and data for submission to FDA, payment of user fees associated with 510(k) submissions, and responding to questions and requests for additional information from FDA during 510(k) review. The surgical light exempt classification is 21 CFR section 878.4580, product code FSQ. In summary, prior to this exemption, the MezLight would have been subject to a process that costs on average $31 million prior to manufacture and sale. Now the only pre-sale requirement is for the registration of manufacturer and listing of product(s) prior to manufacture and sale, which costs $5,236 in annual fees; and ongoing compliance with medical device labeling, good manufacturing practices (GMP) and unique device identification regulations.
Also, at the same time, several reports fed our enthusiasm for MezLight, including:
1. The depressing report that 68% of surgeons performing open surgery after 10 years suffer from some form of disability due to neck and back issues; and
2. A report on bacteria contamination from wiping down the headlight during surgery.
We filed for a patent for the light; we hired a team to help us get off the ground while we tended to our full time jobs; and we raised $1M of initial investment at a $20 million independent valuation, based on our business plan and pro forma projections. The initial investment came from friends and family and proved to be quite easy as they assessed the opportunity. We designed an amazing marketing program using the Frantz Group out of Milwaukee to help get our messaging on target. We built and launched our website, built an amazing trade show booth and attended our first tradeshow, the American Transplant Congress (ATC) show in Boston in June, 2019.
The June trade show allowed us to “soft-launch” the product. We learned a ton and immediately tasked our head of design, Bill Dorr, to pull off a redesign in 5 months so we could be ready for the even larger trade show ACS Clinical Congress…for all general surgeons held in San Francisco Oct 27th through Oct 31st 2019.
Now today is Nov 1, 2019 and the MezLight team came back from the ACS trade show in San Francisco last night. Here’s where we are:
Design
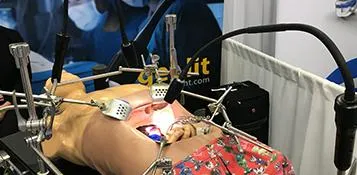
The MezLight has been frozen in major design and is preparing for production. The last small piece to be finalized is the lens configuration, which we expect will be finalized this month.
Our newly chosen product manufacturer, Bridgmedica, located just outside Boston in Mansfield, significantly helped us have the final prototype ready for the ACS show last week. In doing so, they have already identified some design tweaks that allow for a stronger product. Even better, using their 30 years of medical device design and manufacturing experience, they will continue to identify changes to allow them to produce the product better with less expensive production costs. In many cases it’s a change to a tooling for a part to make the tool and/or the part more durable.
For example, we had prepared 7 MezLights for our demonstration use during the show. We all were extremely surprised at the new design strength. We only used the two prototypes for the entire show and we used them hard. This experience bodes well for future testing and validation.

Design & Manufacturing
Since our last update Bill has worked closely with the team at Bridgemedica to evaluate and update the design for cost, performance and manufacturing considerations. This has included mechanical and electrical system updates and has resulted in a truly robust, high quality design solution. Comprehensive design documentation for release to our tooling and parts vendors is being completed this month while we simultaneously fabricate and evaluate a set of final prototypes that capture our changes and revisions. This parallel effort is helping to streamline our development and commercialization timeline. '
In a parallel effort, our optics supplier Lumenflow has tooled the final silicone lens parts, which are a critical element to the system performance, and has tested and verified that we are at or exceeding our lighting performance.
Packaging samples have been submitted by several vendors and are being evaluated by the team with very favorable price quotes. The final packaging design decision is pending the results of shipping survivability testing.
Finally, Bridgemedica has transitioned all documentation into their ISO13485 certified quality management system. Bridgemedica will now be creating and managing all QMS documentation as we move closer to production and FDA registration.'
Last week Heidi, Bill and Craig met with David Casimir to review our US Patent in prosecution. Bill shared some final design changes, and David agreed our patent filing should incorporate additional design elements. So, David will soon be filing a Continuation-In-Part (“CIP”) patent action to add what we are tentatively calling the MezLink. David estimates this CIP will improve our odds of obtaining a patent by 25%. Also, David encouraged the MezLight team to use the labeling “patent pending” on the various products and features of the MezLight system under the claims of the patent application.
Heidi and David will also follow-up to assure MezLight the benefit of trademarks for product names, attributes, and branding elements. So, in addition to “patent pending” you will see TM labeling names like MezLight, MezPost, MezPower, MezLink, MezKit, and phrases like “get lit”.
Sales & Marketing

We have been preparing for the trade show season which for us starts first week of April. We have budgeted for 4 tradeshows so far this year. I made a call on Mayo Clinic and got an agreement to try out the MezLight. Josh has provided us with a very clear list of his friends and supporters that are willing to try the product. Heidi and I will be contacting all of them to arrange an in person visit early this year and to start understanding the process we need to follow to get each doctor to be able to try MezLight in the OR. We also have our list of over 200 tradeshow leads of those surgeons who want to try the MezLight.
Heidi has acquired mannequins created to demonstrate MezLight’ s application in neck and hand surgery. This will be especially relevant for the AAES event in Birmingham, in which thyroid procedures are prevalent in this specialty.
In addition, Heidi has been working with the Value Assessment team at UW Hospital to get us into the UW OR as fast as possible. To date there has been positive progress and they are very excited with the potential for our UW surgeon-created medical device.
What is the opportunity?
You’re receiving this letter and investor presentation because you’ve said you’d be willing to consider an investment in MezLight. I did include friends who had looked at us before simply due to our exciting updates and new business plan goals.
The MezLight, under my leadership, will be the industry standard for surgeon-controlled lighting in the OR by removing the headlight and liberating the surgeon.
If you are inclined to invest, please let me know and I’ll send to you our private placement memorandum (PPM) which has more detail and investment instructions.
I’ll reach out to you personally to follow-up. In the meantime, please feel free to contact me at 608-712-1165 or [email protected]
With gratitude,
Karen Christianson
MezLight CEO & Co-Founder
To access the MezLight presentation click below!

525 Junction Road
Suite 6500
Madison, WI 53717
f 608.960.4610

Copyright 2025. MezLight. All Rights Reserved.